Pharmaceutical Microbiology: The 4 Best Culture Media Suppliers for Environmental Monitoring
Choosing a supplier for culture media in environmental monitoring is a bit like getting married… You hope it will last a lifetime, but sometimes it ends in divorce.
And that’s when things get messy… because everything will need to be revalidated!
The first step is to create a requirements specification, and you can find all our tips in this article “How to choose the best culture media for Environmental Monitoring in pharmaceutical microbiology?.”
Next, you’ll need to start the search for a new supplier, and this is where the “Super Power” of SuperMicrobiologistes comes in handy. Our superpower is the 20,000 microbiologists who regularly follow us. Your feedback is worth its weight in gold.
We surveyed the SuperMicrobiologistes to find out which culture media suppliers are most appreciated.
The result? 4 brands stand out. Here they are:
Content :
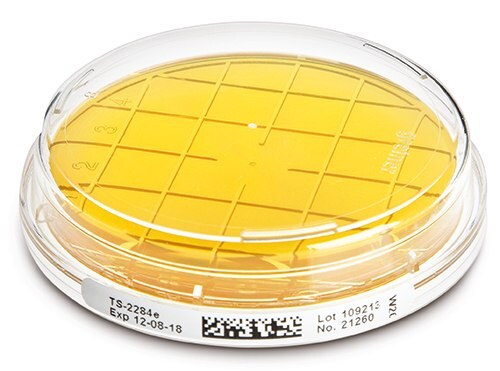
ICR / ICR Plus culture media from Merck
Merck offers a wide range of culture media dedicated to microbiological monitoring in aseptic production environments (clean rooms, RABS, and isolators).
The culture media, available in contact plates, Petri dishes, and swabs, are gamma-irradiated, triple-wrapped (impermeable to H2O2), and contain neutralizers.

Key advantages of ICR (Isolator Clean Room) culture media:
- A wide range of neutralizers (to be selected based on the disinfectants/antibiotics used in production).
- Storage at ambient temperature or cold (4°C – 25°C).
- Shelf life of 12 months from production for TSA (Tryptic Soy Agar)
- A 30 ml fill volume.
- Sabouraud media are packaged in pink plates to differentiate them from TSA plates.
Key advantages of ICR Plus culture media:
- A 2-position locking system: “closed” and “vent” for anaerobes.
- A more comprehensive certificate of analysis, including:
- Verification of anaerobic growth in plates (Clostridium sporogenes).
- Disinfectant neutralization tests.
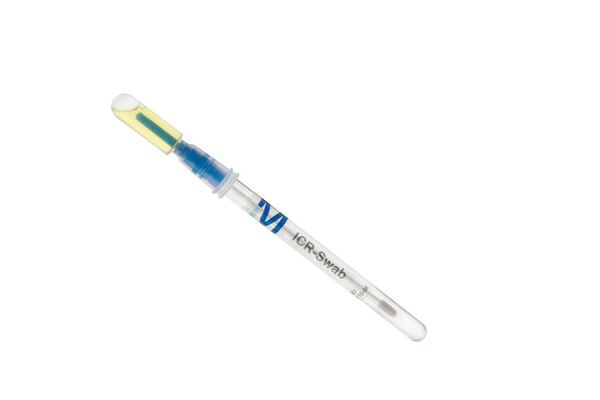
Key advantages of ICR Swab:
- An integrated culture broth reservoir that reduces sampling to a single step, minimizing the risk of secondary contamination: designed for presence/absence results in classified areas A, B, or similar.
- Pre-moistened for convenience.
- Unique barcode for easy traceability.
CSG culture media from PMM

PharmaMedia Dr. Müller (PMM) was founded in 2013 and has specialized on the production as well as the development of microbiological media for all kind of applications in the pharmaceutical industry.
PMM produces them in clean rooms that fulfill most of the requirements of a grade A area surrounded by a grade B clean room. As a result, around 75 million plates have been produced throughout the last 10 years, without any observed contamination (numbers given by the supplier… we haven’t verified them one by one !).
Product portfolio comprises environmental monitoring media, bottled media and a All-in-One swab. In addition to standard products, PMM offers customized products according to customer requirements and needs.
All PMM products are provided with a unique identification barcode, allowing to easily identify each single container.
Key advantages of the PMM EM media:
- Produced under strict pharmaceutical conditions.
- All plates used at PMM contain a locking lid system, which provides incubation options optimized for the detection of either aerobic or anaerobic microbial count.
- 12 months shelf-life from day of production for TSA and SDA media.
- SDA media are filled in colored plates to facilitate the differentiation from TSA plates, both during application and incubation.
- Standard 30 ml filling volume for sedimentation plates.
- TSA-U+, a special neutralizer contact plate is available to neutralize residues of non-volatile disinfectants, like quaternary ammonium compounds (QACs), which may be taken-up when performing contact tests.
New in 2025: Contact plates will be provided with the new Improved Condensation Control Design (ICCD), thereby reducing the risk of condensation collection outside the plates.
Key advantages of the PMM Selective Media for testing non-sterile products
PMM range of selective media covers most of the media required for testing non-sterile products. These include TSA, SDA, MacConkey-Agar, Mannitol-Salt-Agar, Cetrimide-Agar, VRBD-Agar and XLD‑Agar as well as a medium for Burkholderia cepacia complex.
- The majority of the media is provided with a long shelf-life of 8 or 9 months (except for XLD-Agar with a shelf-life of 4 months).
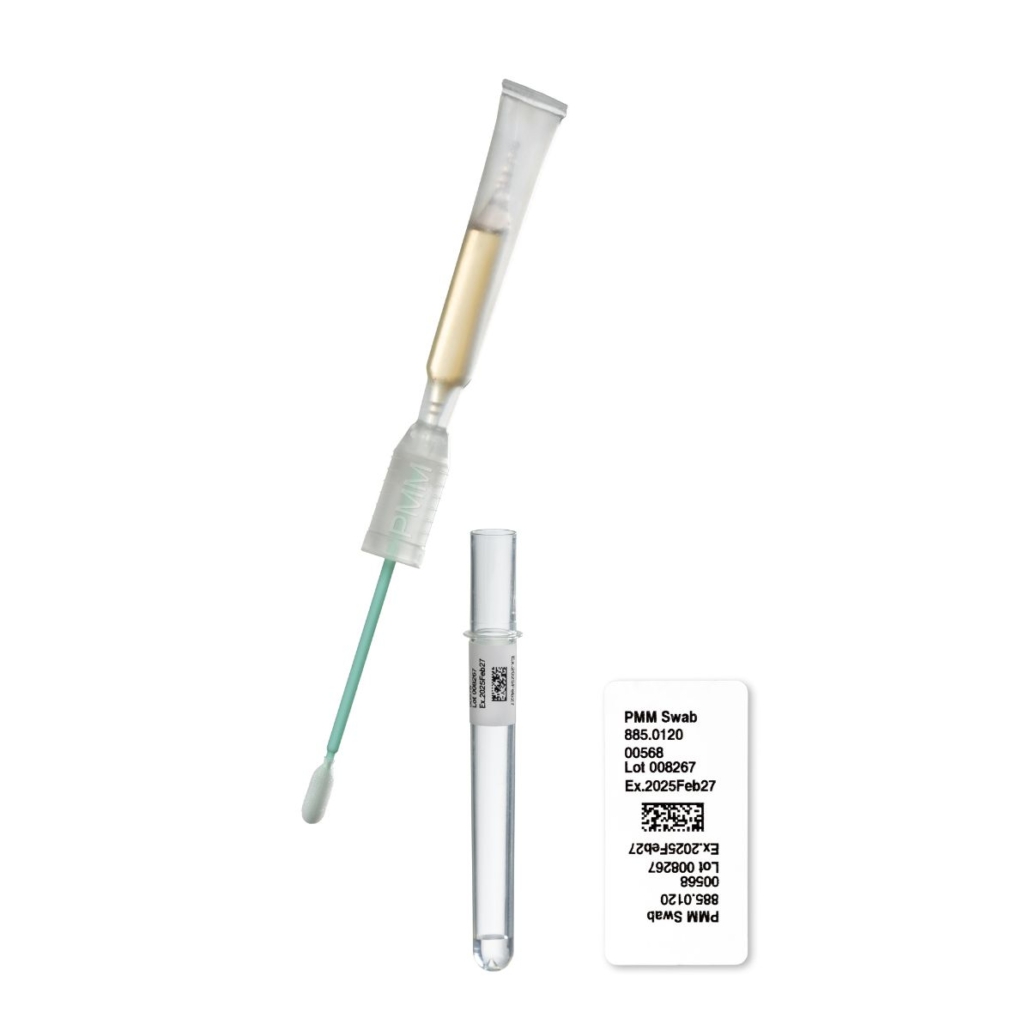
The PMM All-in-One swab are
The PMM All-in-One swab has been recently developed to sample the most critical areas of a pharma production that cannot be easily accessed with contact plates.
This Swab is optimized to provide a qualitative test (presence/absence) for microbiological contamination.
The All in one Swab from PMM is made of:
- A flat-sized swab paddle pre-wetted with physiological NaCl solution.
- The growth medium – TSB medium containing neutralizers to neutralize disinfectants potentially present on the sampled surface
- The growth medium in the PMM All-in-One swab is filled in an ampoule and autoclaved in the final container, thus avoiding an aseptic filling process of the medium into the device. Furthermore, the swab itself is gamma-sterilized applying a doses of >25 kGy.
The other media mentioned by the SuperMicrobiologistes include the 3P range from bioMérieux, the range from BD.
Why are some brands featured more prominently than others?
Following the survey conducted with the SuperMicrobiologistes, we reached out to all the brands that were highly recommended to offer them the opportunity to sponsor this article.
This sponsorship allows us to provide you with all our content for free.
The brands that are “better” presented are those that agreed to support SuperMicrobiologistes by sponsoring the article. We thank them for their support.
So, if you’re looking for a culture media supplier, start by testing these brands!













Leave a Reply
Want to join the discussion?Feel free to contribute!