Microbiology Pharma: The Best Rapid and Alternative Methods
We asked the SuperMicrobiologists (over 13,000 followers on LinkedIn) which rapid (or alternative) methods they use in their microbiology laboratories.
Here are all the methods that were reported back to us. The choice is yours!
Content :
- Red One by Redberry
- IAN by Microbs
- Milliflex Rapid by Merck
- ScanRDI by bioMérieux
- BactAlert by bioMérieux
- The challengers
Take part now in the Survey for 2025
Red One by Redberry
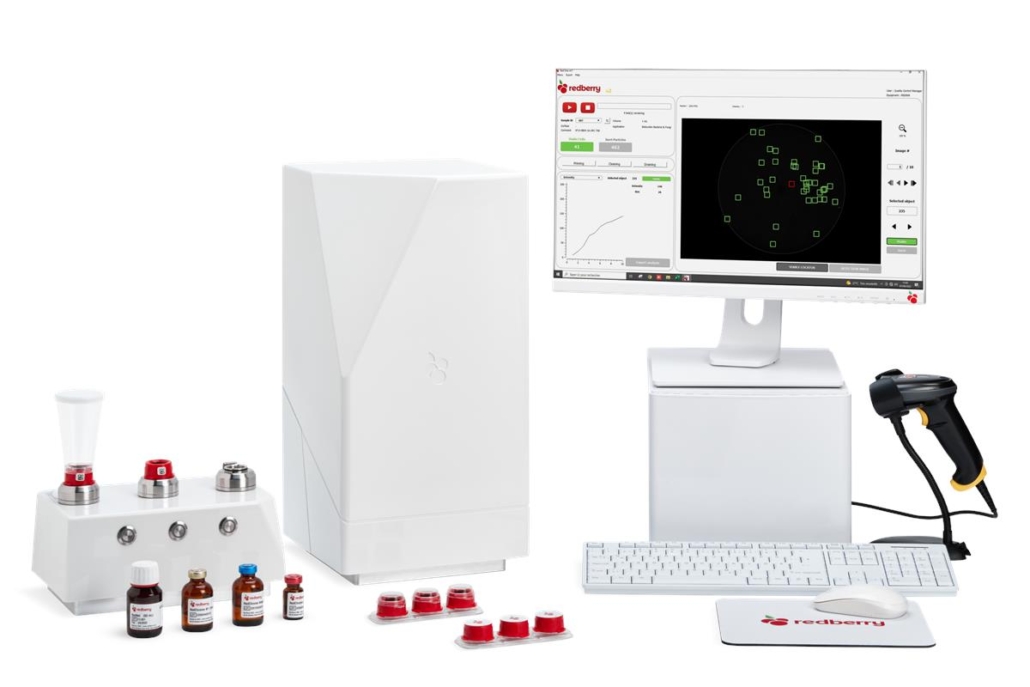
Red One™ is the new generation of automated solid-phase cytometer, enabling rapid and automatic enumeration of microorganisms.
It analyzes the real-time assimilation of fluorescent markers by cells (kinetic marking – Redberry patent) to provide an accurate count of the number of microorganisms in samples ranging from 100μL to 100 mL.
Tests available
- A rapid and quantitative test for total bacterial load in 10 minutes, useful for environmental controls of water and surfaces, strain bank enumeration, mycobacteria enumeration, and monitoring the reduction rate after disinfection.
- A quantitative bioburden test in less than 4 hours for bacteria, yeasts, and molds in process waters, in-process products, and finished products.
- A sterility test compliant with regulatory requirements in 96 hours, with no change in the preparation method: LOD = 1 CFU after enrichment in FTM and SCDM media (primary validation according to Eur. Ph. 2.6.1 in progress).
- Focus on ATMP : Protocols for cellular matrices include a selective lysis phase prior to analysis. Thus, Red One™ offers three levels for controlling cell therapy products:
- Rapid screening in 10 minutes: immediate results and reduced contamination risk.
- Rapid release test in 6 hours (according to Eur. Phar. 2.6.27): same-day results with a detection limit of 100 CFU.
- Sterility test in 96 hours (according to Eur. Phar. 2.6.1): based on the regulatory sample preparation method in FTM and SCDM media (allowing for subsequent identification). LOD = 1 CFU.
- Rapid screening in 10 minutes: immediate results and reduced contamination risk.
Redberry will offer the primary validation package starting this autumn (November 2024) along with support for product validation (protocol, implementation, formalization, possibly with a partner laboratory).
Technology Advantages
- Easy to use: Filtration, marking, and analysis are automated. The device can be used in production or laboratory settings, including under LAF, for analysis within a few minutes (including filtration), even on complex matrices (ATMP, mRNA…).
- Simple to interpret: No operator interpretation required and verification/signature capability (21CFR). The marking technology distinguishes microorganisms from background noise without the need for post-analysis microscopic control.
- Identification: In the event of a positive result, identification using traditional methods is possible.
Technology “Limits”
- The duration of an analysis on the equipment is 10 minutes per sample (from 1 mL to 100 mL), allowing up to 6 samples per hour to be analyzed (including filtration).
- Detection of viable but non-culturable microorganisms is possible in the 10-minute test, which may require regulatory evolutions for complete integration in some cases.
Several users have presented their results at conferences (Pharmig, Pharmalab) for both bioburden (Sanofi) and sterility tests (Eurofins). Other communications are planned by the end of 2024.
Several pharmaceutical users in France and Europe have acquired Red One™ and are currently conducting validations (bioburden on purified water, sterility test).
Contact Redberry : info@redberry.net
Tell them you’re referred by SuperMicrobiologists.
IAN from Microbs

Ian is a solid-phase cytometer coupled with artificial intelligence. This technology allows for the detection and quantification of microbial contaminations in 15 min.
We have limited information regarding the labeling principle. We only know that the implementation of the markers is sequential, which would allow for better discrimination between particles and microorganisms. Optimized formulation to improve spore labeling.
Tests available
TVC (Total Viable Count) for water (bioburden), environmental controls (swabbing), but also finished products (sterility test) in 15 minutes.
Technology Advantages
- Fully automated: Filtration and reagent addition are automated, no manual handling required.
- No expertise required: No interpretation of results. The technology provides a final result in the number of living microorganisms per volume analyzed.
Technology “Limits“
- Analytical throughput: limited to 4 samples per hour.
- Possible detection of viable but non-culturable microorganisms: regulatory evolutions necessary to fully integrate this aspect (method superiority).
Contact Microbs : ian@microbs.fr
Tell them you’re referred by SuperMicrobiologists.
Milliflex® Rapid System 2.0 from Merck*

The Milliflex® Rapid System 2.0 is a rapid microbiology method based on ATP bioluminescence that detects micro-colonies invisible to the human eye… making it faster!
Sample is filtered, and the filter is placed on an agar plate using the Milliflex Oasis® technology, just like for the traditional method.
After a period of incubation (time depends on the test), the plates are sprayed with a specific reagent and then read with a camera that detects the presence of ATP in the living microorganisms’ cells.
The software analyzes the results and provides a colony count per plate.
This technology allows the detection and enumeration of bacteria (including anaerobes), yeast, and mold.
Milliflex® Rapid 2.0 system allows to perform bioburden testing as well as sterility testing with one single equipment
Tests available
- Bioburden test in ¼ time of the compendial method.
- Sterility test in 5 days or less.
Technology Advantages
- Easy to use: Sample preparation is similar to the traditional method. making validation easier!
- No data interpretation: The system provides a result in colony-forming units (CFU), which can be correlated with traditional methods.
- Data integrity: The human eye is replaced by an automatic reader, ensuring all results are automatically saved in a 21 CFR part 11 compliant software.
Technology “Limits”
- Only for filtrable samples
- Microorganism identification is possible (after the detection step and re-incubation on fresh media), but requires to work in aseptic conditions and perform validation
*The Life Science business of Merck operates as MilliporeSigma in the U.S. and Canada.
SCANRDI® from bioMérieux

The SCANRDI® revolutionized rapid microbial detection when it was first introduced. Its speed and sensitivity continue to meet today’s market needs.
Its technology, based on solid phase cytometry, delivers microorganism count results in 4 hours or less, allowing quickly to decide whether to continue production or take immediate action.
The SCANRDI® is a non-growth-based rapid microbial method (RMM) that detects not only viable microbial cells that can be isolated using a broth or agar plate but also viable but non-culturable (VBNC) microorganisms, including stressed and fastidious microorganisms that may not be recovered by standard culture methods.
The protocol involves 4 steps:
- Filtration of the sample (from 1 to 1000ml)
- Labeling of the microorganisms
- Scanning to detect the microorganisms
- Microscope confirmation
Tests available
- Sterility test: in less than 4 hours (down to 1 microorganism/ filtrable volume)
- Bioburden test: in less than 4 hours (from 1 to 5000 microorganism /membrane)
Technology Advantages
- Ultra-rapid : Same-shift results to allow a faster product release.
- Tested & proven technology : This technology has been validated by many pharmaceutical laboratories, for Bioburden as well as for Sterility tests.
- Compliant : Full validation support: URS completion guide, IOQ guide, primary validation guide, suitability guide and Drug Master File (DMF). 21 CFR part 11 enabled software
Technology “Limits”
- Throughput : maximum 20 to 30 samples per day.
- Possible detection of viable but non-culturable microorganisms: regulatory evolutions necessary to fully integrate this aspect (method superiority).
Contact bioMérieux
Tell them you’re referred by SuperMicrobiologists.
BACT/ALERT® 3D from bioMérieux

BACT/ALERT® 3D is an automated growth-based method designed for the early detection of contaminants.
The product is inoculated into a ready to use media bottle. The bottle is then incubated in the BACT/ALERT, which acts as both an incubator and a detector.
If contamination is present, a color change at the bottom of the bottle triggers a lab alert.
Tests available
- Sterility Test: Reduces testing time by half (depending on customer validation).
- In-process Bioburden : Detection of 90% of contaminants between 48-72 hours.
Technology Advantages
- Simple Workflow: Inoculate the bottle and load it into the BACT/ALERT, the rest is fully automated.
- Continuous Monitoring: Growth is monitored every 10 minutes, providing an early alert in case of contamination, allowing for quick corrective actions.
- Matrices Compatibility: Suitable for in-process bioburden or sterility testing of even the most complex matrices such as Cell & Gene therapy products.
Technology Limits
- Inoculated Volume: There are volume limitations for inoculation (10 ml max per bottle)
BACT/ALERT® 3D is considered as a compendial method for sterility testing of Cell & Gene therapy products in China (Ch. 9406) and Europe (EP 2.6.27) and soon to be compendial for short shelf life product in the US… pending the publication of USP 72.
BACT/ALERT® 3D is used by over 700 food and pharmaceutical companies worldwide.
Contact bioMérieux
Tell them you’re referred by SuperMicrobiologists.
The Challengers RMM
7000RMS from Mettler Toledo

The 7000RMS continuously monitors (every 2 seconds) and directly the microbial load present in a water loop.
This analyzer is directly connected to a water loop. Each time particles pass in front of his laser, two optical phenomena are analyzed: fluorescence and Mie scattering (determines particle size). When the signals meet specific criteria at the same time, a micro-organism is present.
This technique not only detects, but also quantifies microorganisms.
Test available
Bioburden in pharmaceutical-use waters: Continuous and real-time.
Technology Advantages
- Continuous monitoring: Contaminations are not always uniform (e.g., biofilms), so taking spot samples can introduce analytical bias. With this technology, the water loop is continuously monitored.
- Immediate results: The detection of the presence of microorganisms is instantaneous. Corrective action can therefore be quickly implemented.
- Optimization of disinfection cycles: This technology allows for quick verification of a water loop cleaning’s effectiveness.
Technology “Limits”
Will the 7000RMS replace filtration and Petri dishes? Maybe not initially. It is seen more as an additional tool to continuously monitor what’s happening in water loops.
Contact Mettler Toledo – Tell them you’re referred by SuperMicrobiologists.
Other RMMs mentioned by the SuperMicrobiologists are: Growth Direct and Celsis.
Why are some brands “better” presented than others?
Following the survey among the SuperMicrobiologists, we contacted all the favored brands to offer them sponsorship of this article.
This sponsorship allows us to offer all our content for free.
The brands “better” presented are those that agreed to support SuperMicrobiologists by sponsoring the article. We thank them.
So, if you need an RMM, start by testing these brands!
What about you? Which RMM have you chosen? Come share your experience in the comments.
















Leave a Reply
Want to join the discussion?Feel free to contribute!