Listeria: What Impact Will the New European Regulation Have on Your Lab?
Do You Produce Ready-To-Eat (RTE) Foods?
Then this new European regulation on Listeria monocytogenes is definitely worth your attention!
But regulations can be tricky to understand.
To make things clearer, we spoke to experts.
Everything you need to know about this new regulation is summarized (and simplified) in this article.
Content:
- Why was the regulatory criterion for Listeria monocytogenes changed?
- Which products are affected?
- What’s changing?
- What does this mean for manufacturers?
- How and where can these Challenge Tests be carried out?
- When does the new regulation come into effect?
Best Lab Blenders
The best lab blender food microbiology
We polled the SuperMicrobiologists.
Here are their favorite lab blenders
Why Was the Regulatory Criterion for Listeria monocytogenes Changed?
On November 20, 2024, the European Commission adopted Regulation (EU) 2024/2895, amending Regulation (EC) 2073/2005.
Here’s a quick backstory: 25 member states voted in favor of this change, Finland abstained, and Belgium voted against.
But why this change now?
Here are the two main reasons:
- An Increase in Listeriosis Cases in Europe1
The numbers speak for themselves, with a significant rise in cases since 2019. - A Legal Gap Identified by the European Court of Justice (ECJ)
In 2022, the ECJ highlighted a loophole in the previous regulation, which could be interpreted differently across countries.
As a result, the ECJ allowed national authorities to enforce stricter measures than those outlined in European regulations.
This new regulation addresses these ambiguities to ensure clearer criteria and less confusion.
Fewer gray areas, more safety (at least, that’s the goal)!
Which Products Are Affected?
The new regulation targets ready-to-eat (RTE) foods that support the growth of Listeria monocytogenes.
These foods are considered higher risk because they aren’t subjected to any sanitizing treatment before consumption, and their long shelf life can allow Lm to grow even when stored at positive cold temperatures.
Exceptions:
- Products specifically intended for infants.
- Foods designed for special medical purposes.
What’s Changing?
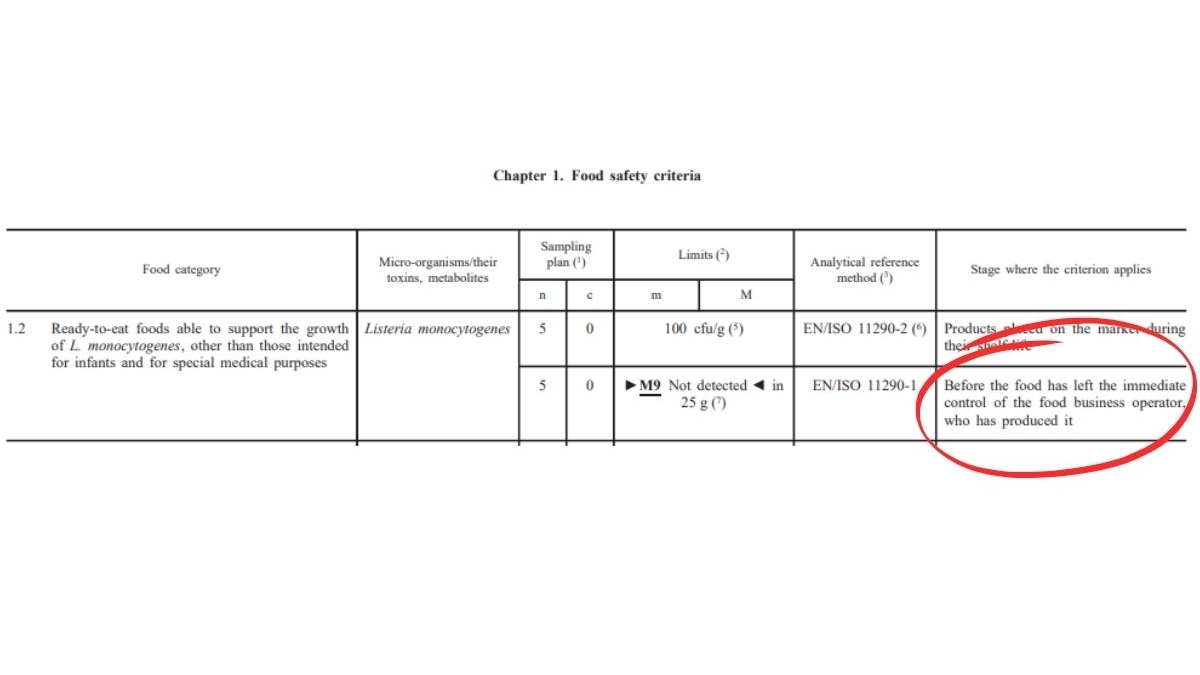
Before (Regulation (EC) 2073/2005)2:
After (Regulation (EU) 2024/2895)3
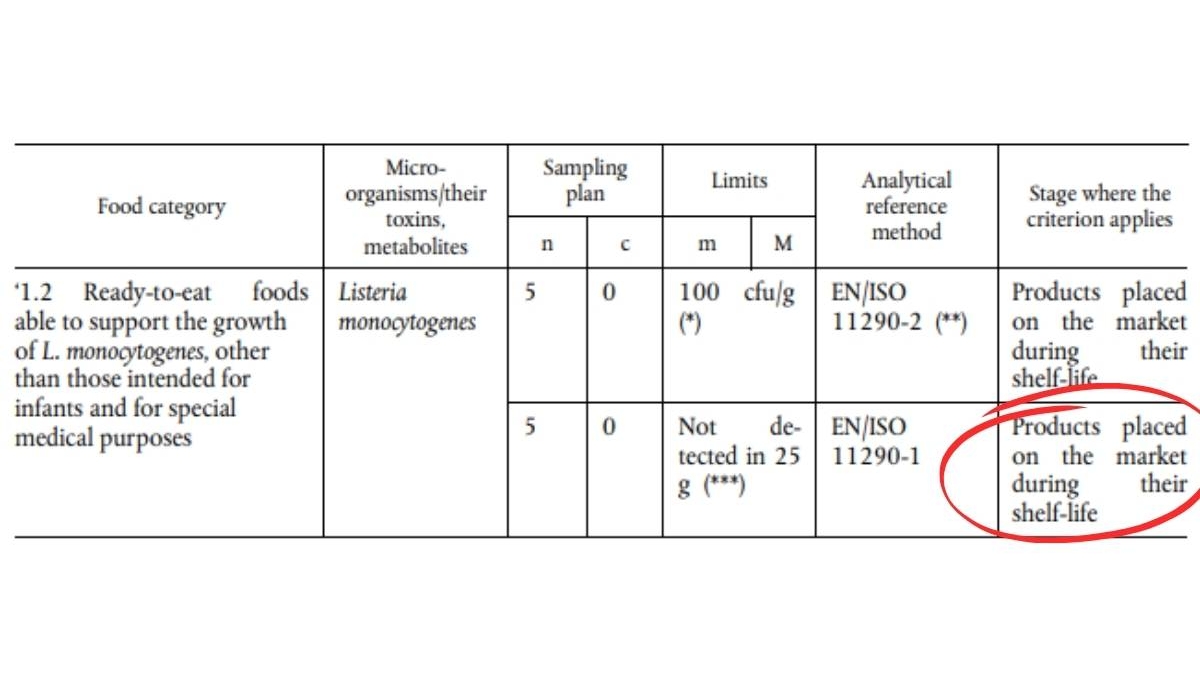
For products where Listeria monocytogenes can grow, manufacturers are now required to prove that the 100 CFU/g limit is respected until the end of the product’s shelf life (DLC).
If the manufacturer cannot provide this proof, the product must meet the stricter criterion of absence in 25 g.
In summary: The tolerance is stricter, and scientific demonstration is now mandatory!
What Does This Mean for Manufacturers?
In some countries, like France, not much will change since they had already addressed the gap in the EU regulation.
But be careful!
If this is the not the case in your country, you’ll need to prove that, in the event of initial contamination with Listeria monocytogenes (Lm), the bacterial count will never exceed 100 CFU/g throughout the product’s entire shelf life.
To demonstrate this, several tools are available to validate and/or verify the shelf life of an RTE product. These tools are listed in the regulation, particularly in the technical instruction from the DGAL (DGAL/SDSSA/2004-270).
Among these, you can use:
- Predictive Microbiology: Mathematical models that predict bacterial behavior based on factors such as physicochemical parameters, temperature, etc.
- Challenge Tests: Experimental contamination of a product to monitor the growth of Lm over a specific period and in a specific product.
Can Predictive Microbiology Replace a Challenge Test?
The answer is clear: NO.
Predictive microbiology modeling helps anticipate bacterial behavior under various scenarios without needing lab-based analyses.
However, a growth test is essential to gather precise information about bacterial behavior in a specific product under specific storage conditions.
These two approaches are therefore complementary.
In all cases, the reliability of shelf-life studies depends on the relevance and logical application of the various tools available.
In All Cases, It’s Important to Fully Understand Your Product, Including:
- The history of self-inspections
- Manufacturing conditions
- pH
- Water activity (Aw)
- Inhibitory ingredients
- Additives
- Salt content
- Additional microbial flora
- Type of packaging
- …
- And, of course, the shelf life
The 3 best Media Preparator
We polled the SuperMicrobiologists.
Here are their favorite ones
How and Where to Perform Challenge Tests?
A Challenge Test involves artificially contaminating a product and then measuring the quantitative evolution of the contamination throughout its shelf life.
In this case, the product is contaminated with Listeria monocytogenes (Lm), and the test checks that the Lm count does not exceed 100 CFU/g.
Who Can Perform Challenge Tests?
- For Pre-analysis or R&D:
- These tests can be performed in your own microbiology lab, if you have one.
- Alternatively, you can work with a specialized service provider.
- These tests can be performed in your own microbiology lab, if you have one.
- For Regulatory Self-Inspection:
- If the tests are conducted to comply with Regulation 2073/2005, they must be carried out in laboratories recognized by the local authorities to perform growth tests (Challenge Tests).
Optimizing Your Challenge Tests
To reduce the number of Challenge Tests (which can be costly and time-consuming), you can:
- Group Products by Family: Select products with similar characteristics (production process, shelf life, packaging, etc.).
- Test the Highest-Risk Product: For example, if you produce various types of ready-to-eat salads, identify the one with the formulation most susceptible to Listeria monocytogenes contamination.
Bonus Tip
Connect with your professional association: It’s possible that studies on similar product matrices have already been conducted, which could save you time and resources.
Best QC strains
Food & Water : The best QC Strain suppliers
We polled the SuperMicrobiologists.
Here are their favorite QC Strains
Effective Date of the New Regulation
Regulation (EU) 2024/2895 will come into effect on July 1, 2026.
Although this date might seem far off, don’t wait too long!
The required studies can take a significant amount of time to complete, and the number of recognized laboratories is limited.
Thank You to All Contributors
We would like to thank the SuperMicrobiologistes experts who helped us decode this new regulation, review, and refine this article.
A big thank you to Corinne Danan, Ruben Barcia-Cruz, BALON Cecile, Laurence Giuliani, Fanny Le Doeuff, Guillaume Frelat, Alexandre Leclercq, Stéphanie Kwasek and Adrien Asséré.
If you still have questions about this regulation, feel free to ask them in the comments!












Leave a Reply
Want to join the discussion?Feel free to contribute!