Microbiology: 6 Tips for Perfect Pipetting
If you’re on the SuperMicrobiologistes website, chances are you already know how to use a micropipette. So, we won’t go over the basics of pipetting again.
The goal of this article is to help you gain a few extra percentage points of precision so you can become true micropipette pros.
We’ve explored microbiology labs and pipette supplier sites to bring you 6 tips that could make all the difference in your pipetting!
Best Pipettes
The 8 Best Micropipettes
We polled the SuperMicrobiologists.
Here are their favorite Micropipettes
1 – The micropipette is not a hammer
When attaching the tip to the micropipette, it’s better to use a twisting motion rather than slamming it on like a maniac.
This ensures the tip is properly positioned and makes it easier to eject later.
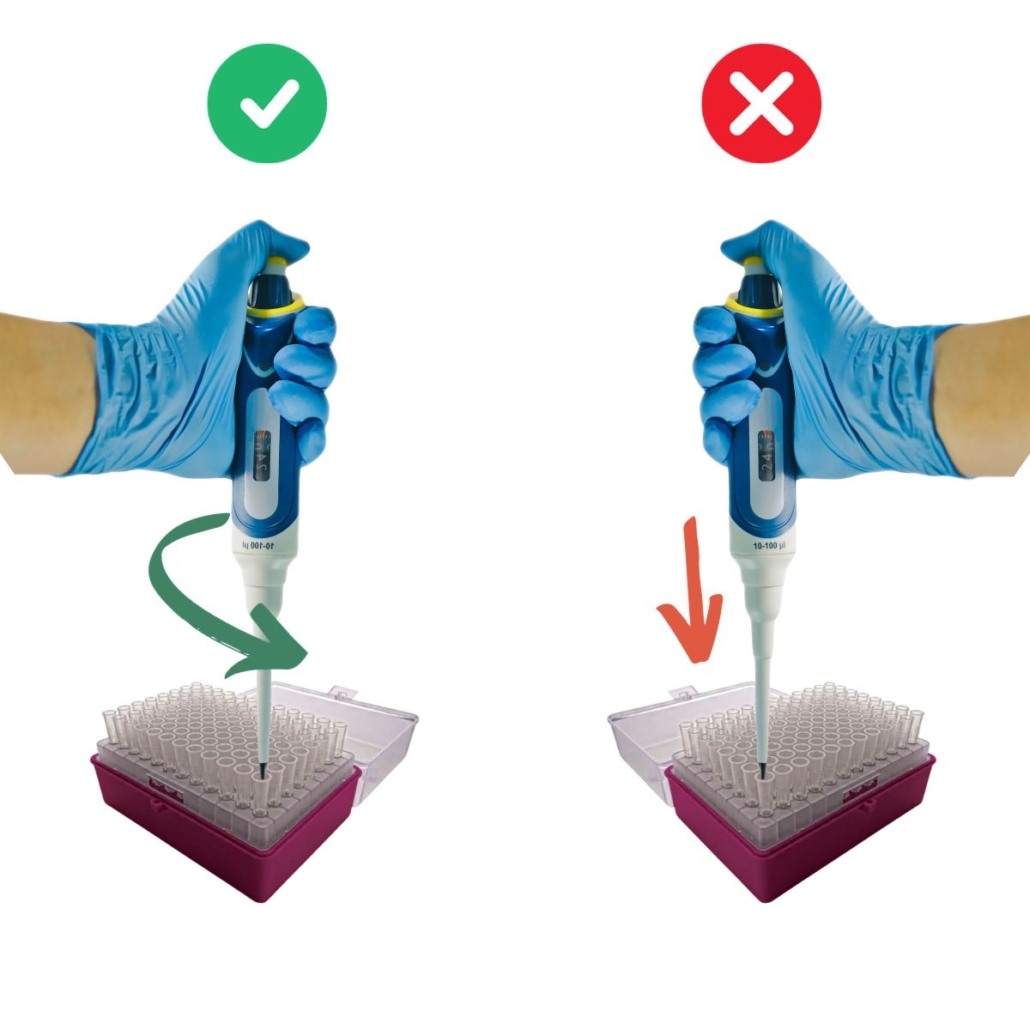
2 – Don’t Dip the Tip Too Deep into the Liquid

When pipetting, avoid submerging the tip too deeply into the liquid. Ideally, the tip should only be 2 to 4 mm below the surface.
There are two main risks to dipping the tip too far:
- The depth increases pressure on the air cushion, causing it to compress and draw in slightly more liquid than intended.
- Liquid may stick to the outside of the tip.
For the best results, keep the pipette perpendicular to the liquid during aspiration. The more you angle the pipette, the less liquid you’ll actually draw compared to the intended volume. Keep the angle under 20°.
3 – For Perfect Dispensing
Dispensing a liquid with a micropipette into a tube
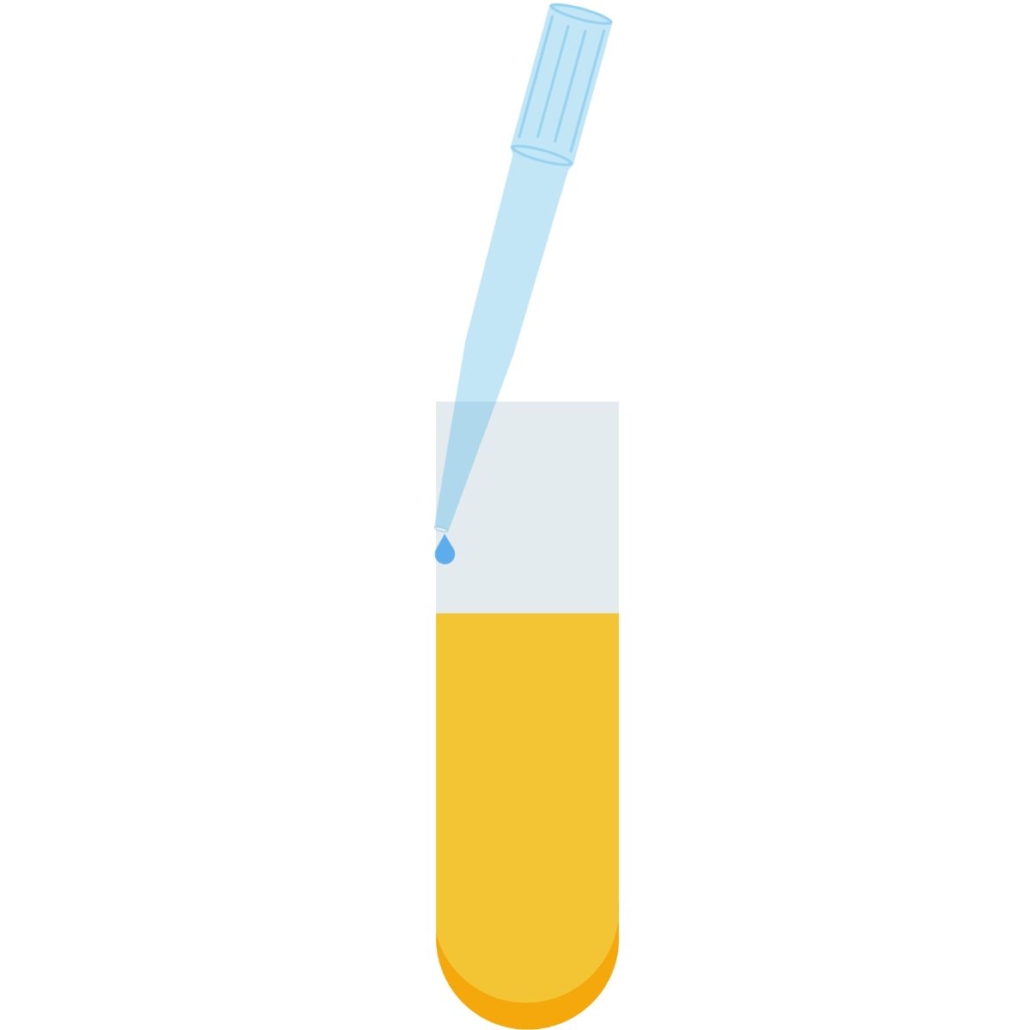
When dispensing liquid into a tube with a micropipette, aim to distribute it along the wall. The tip should gently bend as it touches the side of the tube.
This ensures the tip is emptied completely, which is especially important when working with small volumes.
Dispensing with a micropipette in a Petri dish
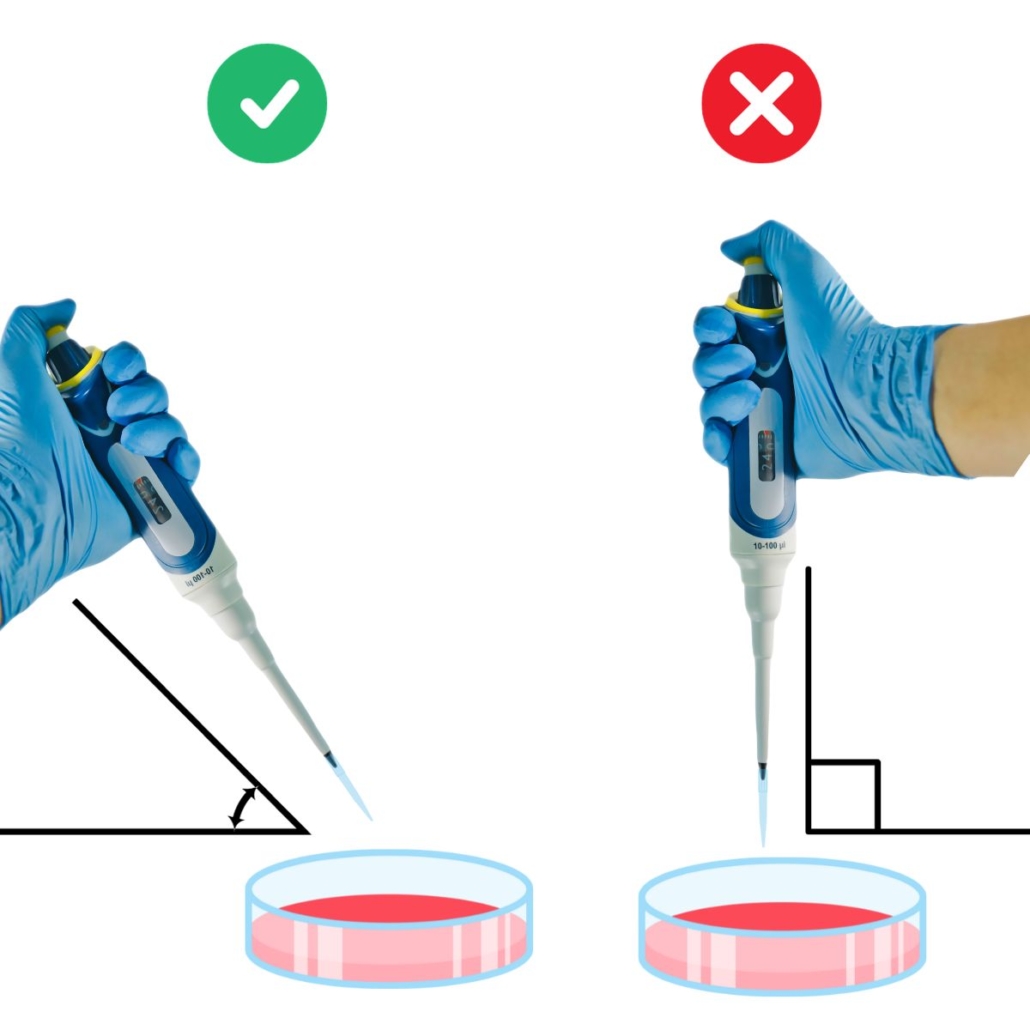
When dispensing into a Petri dish, avoid holding the pipette perpendicular to the dish.
Instead, aim for an angle of about 45° (no need to bring out a protractor). Dispensing at an angle helps reduce the risk of splashing or aerosol formation.
Also, make sure not to touch the surface of the agar.
4 – Go Easy on the Plunger
Whether you’re aspirating or dispensing liquid, avoid using abrupt or forceful motions with the plunger (piston).
The risk? Going too fast can cause bubbles during dispensing or make the pipette “drink” liquid during aspiration.
And a pipette that “drinks” is never a good thing!
5 – Pre-Wet Your Tips
Who actually pre-wets their tips?
That’s exactly the question we asked the SuperMicrobiologistes community on LinkedIn!
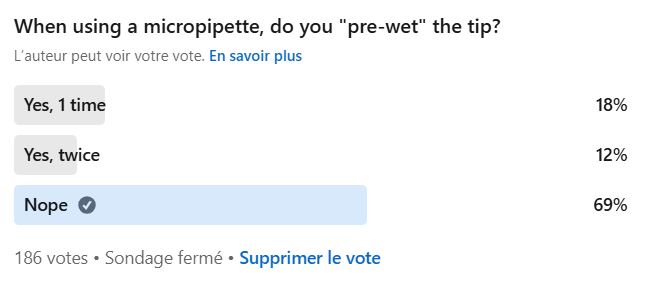
69% of SuperMicrobiologistes Don’t Do It
Surprising, right?
Yet pre-wetting is actually mandatory according to the NF EN ISO 8655-2 standard (Piston-operated volumetric apparatus – Part 2: Pipettes). Not so funny anymore, huh?
To pre-wet the tips, simply perform 2 to 3 cycles of aspirating and dispensing the liquid into the tip.
This step helps balance the temperature between the micropipette’s air cushion and the liquid, ensuring both accuracy and precision in your pipetting.
6 – Avoid Temperature Fluctuations
Ideally (though not always possible), the tip, pipette, and liquid should all be at the same temperature.
The micropipettes we use in microbiology labs are air-cushion pipettes (see article). The air cushion ensures the precision of the pipetted volume.
If you’re pipetting a cold liquid, the air cushion contracts, leading to more liquid being drawn than intended.
On the flip side, with a warm liquid, the air cushion expands, resulting in less liquid being drawn than intended.
Bonus Tip 1: For Variable Volume Micropipettes
With variable volume pipettes, avoid working below 35% of their nominal volume.

While these pipettes can technically handle volumes ranging from 100% to 10% of their nominal capacity, their accuracy and precision significantly drop when you go below 35%.
Bonus Tip 2: For Viscous or Volatile Liquids
When dealing with non-aqueous or foamy liquids (e.g., glycerin, ethanol), use reverse pipetting.
It’s much more precise and can be done with any micropipette. We even wrote a full article about reverse pipetting!
If you have other “tips”, feel free to share them in the comments.













Leave a Reply
Want to join the discussion?Feel free to contribute!