Pharma Microbiology: What is the Role of Neutralizers in Environmental Monitoring Culture Media?
Neutralizers are commonly used in pharmaceutical culture media for environmental monitoring. TSA+LTHT (Lecithin, Tween 80 (Polysorbate 80), Histidine, and Thiosulfate) and TSA+LT are considered standard for neutralization.
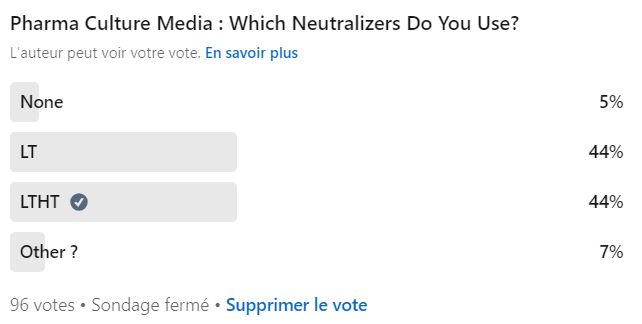
However, the exact types and concentrations of each neutralizers are not specified in either the European Pharmacopoeia (EP) or the United States Pharmacopeia (USP).
This expertise in formulating culture media depends largely on the manufacturer of the ready-to-use media.
In this article, we will explain the role of neutralizers and how to select the appropriate ones.
Content:
- Regulations and neutralizers
- The challenge of neutralizing quaternary ammonium compounds
- A new generation of neutralizers
Best Culture Media
Discover the 4 BEST Culture Media Suppliers
We survey the SuperMicrobiologists.
Here are their favorite EM culture media.
What do the regulations say about neutralizers for environmental monitoring?
Regarding the addition of neutralizers, the European Pharmacopoeia EP 2.6.12 [1] (USP <61> in the US) recommends certain ones. The table “Common neutralizing agents for interfering substances” lists the active ingredients commonly used in disinfectants and their potential neutralizers.
The table below lists the neutralizers recommended based on the disinfectant used:
| Désinfectants / Neutralisants | Sodium bisulfite | Dilution | Glycine | Lécithine | Tween | Thiosulfate | Mg2+, Ca2+ |
| Glutaraldéhydes | X | ||||||
| Mercures | X | X | |||||
| Phénols | X | ||||||
| Alcools | X | ||||||
| Aldéhydes | X | X | X | ||||
| Sorbate | X | ||||||
| QAC | X | X | |||||
| Parabènes | X | X | |||||
| Biguanides | X | ||||||
| Iodine | X | ||||||
| Halogènes | X | ||||||
| EDTA | X |
Table 2.6-12-2 of the EP does not specify the exact concentrations of neutralizers to add. This information could come from the disinfectant manufacturers, who must provide data on the potential inactivation of their products. However, these tests are typically done with concentrations that don’t apply to ready-to-use media. The typical neutralizer concentrations in agar media are as follows: Lecithin (0.07%), Tween 80 (0.5%), Histidine (0.05-0.1%), and Na-Thiosulfate (0.005-0.05%).
While histidine and sodium thiosulfate are well-defined chemicals, tween and lecithin are not. Their biological origin leads to variations in quality, which can significantly affect the neutralizing performance of culture media if not carefully verified before routine use.
The challenge of neutralizing quaternary ammonium compounds (QACs)
Disinfectant formulations evolve over time. Manufacturers continuously innovate to offer more effective products, which can challenge the ability of culture media to neutralize active residues. Some newer formulas are no longer fully neutralized by TSA-LTHT.
The most recent examples are the quaternary ammonium compounds (QACs), which are primarily effective against Gram-positive bacteria.
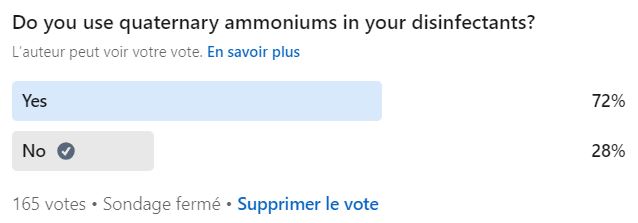
Recent studies1 show that certain QAC formulations, both at low and high concentrations, were not completely neutralized by LTHT. Aware of this issue, manufacturers have developed specific media that neutralize QAC-containing disinfectants more effectively than standard TSA+LTHT.
Even though LTHT is the default, the Pharmacopoeia list is only a recommendation. Alternative neutralizers must meet two criteria as stated in EP 2.6.12:
“Neutralizing agents may be used to neutralize the activity of antimicrobial agents (Table 2.6.12.-2). They may be added to the chosen diluent or the medium, preferably before sterilization. If used, their efficacy and lack of toxicity for microorganisms must be demonstrated2.”
Thus, pharmaceutical laboratories are free to use alternative neutralizers, as long as they comply with regulatory texts, ensuring the neutralizers are non-toxic and effective against the disinfectants used.
A new generation of neutralizers effective against QACs
The table below shows an excerpt from a study on TSA-U+, developed as an alternative neutralizer to better inactivate some of the newer QAC-containing disinfectants.
Each contact plate (TSA-LTHT and TSA-U+) was inoculated with a disinfectant amount equivalent to 20 ml/m². After 15 minutes, test strains were inoculated in each plate and incubated for 18-24 hours for bacteria or 3-5 days for yeasts/molds.
The recovery rate (in %) for TSA-LTHT and TSA-U+ was calculated relative to untreated plates.
| TSA-LTHT | TSA-LTHT | TSA-LTHT | TSA-U+ | TSA-U+ | TSA-U+ | TSA-U+ | TSA-U+ | TSA-U+ | TSA-U+ | ||
| Désinfectant | [QAC] mg/100 ml /[Biguanide]mg/100 ml | S.aureus | B.subtilis | S.epidermidis | S.aureus | B.subtilis | S.epidermidis | E.coli | P.aeruginosa | C.albicans | A.brasiliensis |
| Gigasept AF 4% | 876 / 0 | 0 | 0 | 0 | 92 | 76 | 83 | 116 | 97 | 123 | 99 |
| Hexaquart forte 2% | 558 / 0 | 0 | 0 | 0 | 86 | 106 | 79 | 92 | 101 | 97 | 108 |
| Klercide Quat/Biguanide | 500 / 20 | 0 | 0 | 0 | 111 | 109 | 126 | 105 | 95 | 112 | 89 |
| Amphospray 41IP | 109 / 96 | 0 | 0 | 0 | 87 | 93 | 89 | 85 | 127 | 127 | 119 |
In TSA-U+ plates, thiosulfate was replaced by U+ as an alternative neutralizer. U+ proved to be a highly effective inactivator of QACs and biguanides, without inhibiting microorganisms, as required by EP/USP.
Conclusion: Always validate the effectiveness of neutralization
In every case, whether LTHT or another formula, neutralizers must be validated before use. You’ll need to test them using the site’s cleaning procedures (with all disinfectants), incorporating both EP/USP strains and the environmental flora to reflect real-use conditions.
Article in collaboration with PMM (PHARMAMEDIA DR. MÜLLER)
If you have any questions or comments after reading this article, feel free to join the discussion below.













Bonjour et comment neutraliser l’activité anti microbienne dans un produit fini ( forme sèche) si il le contient comme les antibiotiques par exemple comment exploiter le tableau des agents neutralisant dans ce cas.
Merci
Bonjour Mounira,
La neutralisation des antibiotiques dans les formes sèches est une vraie difficulté pour les microbiologistes ! Il faut jouer avec tous nos atouts (même si on en a pas des masses…) : Dilution, Filtration, Rinçage, et Neutralisation (en utilisant les même neutralisants que ceux du tableau, à différentes concentration).
Certaines formes ne sont pas “neutralisables”, dans ce cas cela permet de justifier que rien n’y pousse 😉
Hi Experts,
Which neutralizers shall be used in TSA and SDA culture media where antibiotics are handled and what concentration? Especially Amoxycillin, Kanamycin, Cephalexin etc. How to implement?
Your valuable guidance is appreciated.
Thanks