How to choose the best method for detecting STEC (Shiga Toxin-producing E. coli)?
There are fewer than ten validated methods for detecting pathogenic STEC in foods… but that doesn’t make the choice any easier.
Some of these methods will be more or less suited to your matrices and your laboratory. It’s therefore important to fully understand the stakes before diving into one technology or another.
The goal of this article is to clear up the subject as much as possible to help you find THE method most suited to your environment.
Content :
- What is a STEC?
- What are the challenges in detecting a STEC?
- Criteria for choosing a STEC detection method
- How to choose your supplier ?
The 6 best PCR for STEC
The 6 best method for STEC detection
We surveyed the SuperMicrobiologists,
here are the top-rated methods
What is a STEC?
A STEC is an Escherichia coli strain that produces shigatoxines. To produce shigatoxine, those strains need to have an stx gene.
But having the stx gene does not means the bacteria will express it and will be harmful… You finally understand why STEC are so complicated 😉 !!
In some countries, where the prevalence of STEC having stx gene is high, to avoid wasting non harmful food, they go further in the description of what is a pathogenic STEC.
Example : E.coli having stx and eae genes are considered as pathogenic STEC.
But still, not all bacteria having eae and stx genes are pathogenic. This is why some countries added another criteria based on E.coli strains which are most frequently involved in human contaminations.
Example : In France, pathogenic strain are E.coli having stx, eae genes and are part of the big 5 (O157:H7, O26:H11, O145:H28, O103:H2, and O111:H8).
So the first step is to better understand what are your local regulations or recommendations.
What are the challenges in detecting a STEC?
If you consider as pathogenic STEC all E.coli strains that possess the stx gene, then it’s pretty straightforward… a simple PCR will give you the answer.
Where it gets more complicated (or interesting) is if you consider as pathogenic an E.coli strain that possesses all 3 genes (stx, eae, and O group).
When performing a PCR, we lyse the bacteria to extract the DNA, during which all DNA strands from all the bacteria are mixed. If the PCR detects the 3 genes, who can say that these 3 genes come from the same bacterium? We consider this result as a presumptive positive.
To know if the sample is truly positive, a confirmation needs to be done by isolating the bacteria on agar plates, and then performing a PCR on each colony to confirm the presence of the 3 genes. The real challenge of STEC detection methods is to reduce the number of presumptive positives.
Quite a challenge for the PCR suppliers!
11 Criteria for Choosing a STEC Detection Method
All methods will have their strengths and “weaknesses”. There are no “bad” methods, just methods that will be more or less well suited to your laboratory.
Let’s now review these criteria.
1 – Avoiding False Negatives
The most important thing for a microbiological quality control laboratory is to avoid false negatives.
Opting for a validated method does not 100% protect you from this kind of problem (which is often multifactorial), but at least you are giving yourself the best chance by using a method known for its performance.
In Europe, methods are certified by AFNOR or Microval (they are more or less equivalents). In the US, methods are certified by AOAC.



A method is only validated if it shows superiority or non-inferiority to the ISO method. It’s therefore a real mark of quality.
(You can use a “non-validated” method, there are several. This doesn’t mean the method is bad, just that it hasn’t been validated by a certification body. In this case, you must conduct your validation to ensure this method correctly detects STEC (it’s a lot of work and responsibility… few labs embark on this kind of challenge).)
2 – The Validation File
On the accreditation bodies’ websites, you’ll find the names of validated methods and their validation files. It’s worth taking a look, at least to check that matrices similar to yours have been validated.
Of course, this won’t exempt you from performing an “internal” validation following ISO 16140 (Food microbiology – Protocol for the validation of alternative methods). Given that all matrices have their specificities (interfering flora, pH, % fat, etc.), this validation will, among other things, allow you to verify that your matrices do not interfere with the alternative method.
Advice: Choose a method that has been validated by AFNOR or MicroVal or AOAC for your matrices (or similar matrices).
3 – Reducing the Number of Presumptive Positives
(only if you are looking for stx+ AND eae+ AND specific STEC serogroups)
When The PCR detects stx and eae genes, it gives a positive result. However, it does not mean that the 2 genes are coming from the same bacteria. It is called a presumptive positive.
In the case you are looking for a specific serotype (Big 5 or Big 7), it is the same problem. You need to make sure that the genes are present in the targeted serotype.
To reduce the number of presumptive positives, some methods offer solutions.
Pre-PCR Screening Immunocapture
There are different types of immunocaptures that can be done with magnetic beads, referred to as IMS (Immuno-Magnetic Separation) or without. The principle of this immunocapture is simple.
In the context of IMS, magnetic beads are coated with antibodies that are specific to certain E.coli serotypes.
The immunocapture step takes place just after the incubation period of the matrix in the broth.
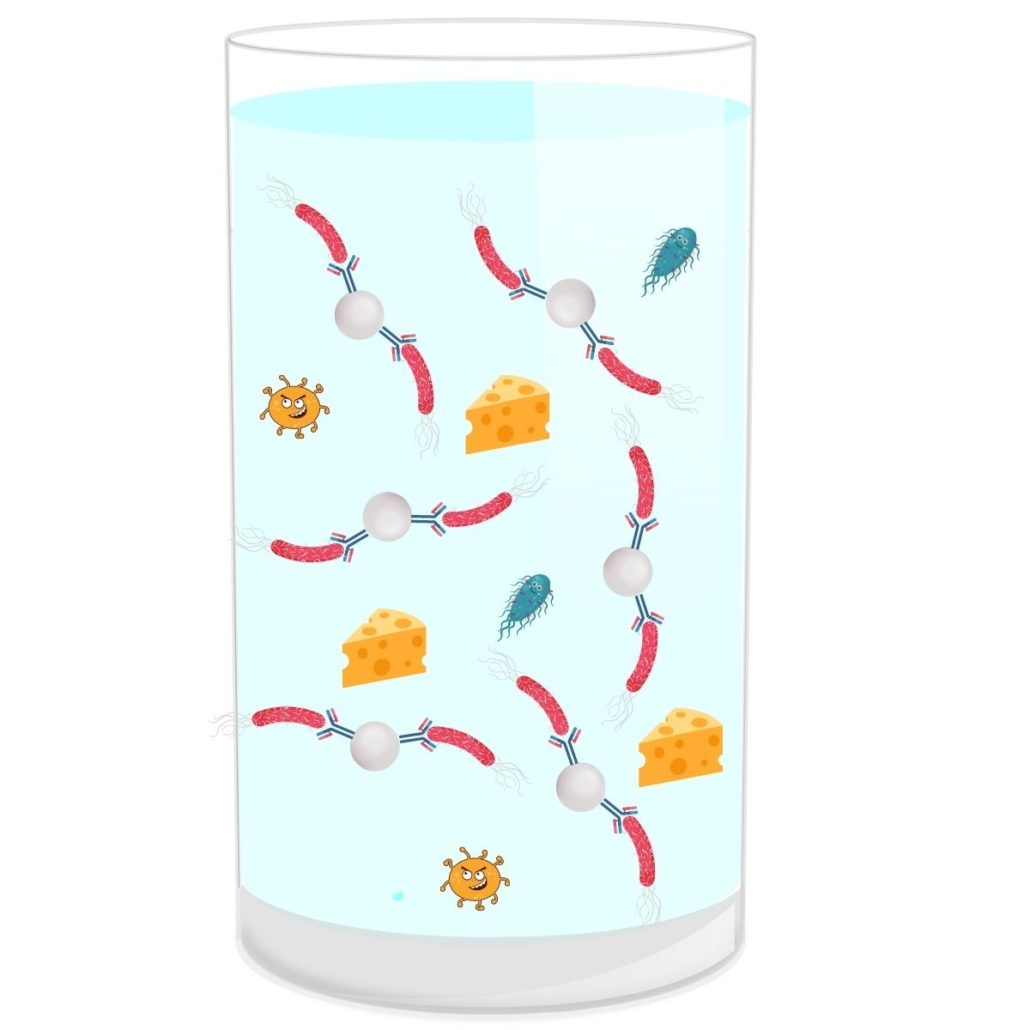
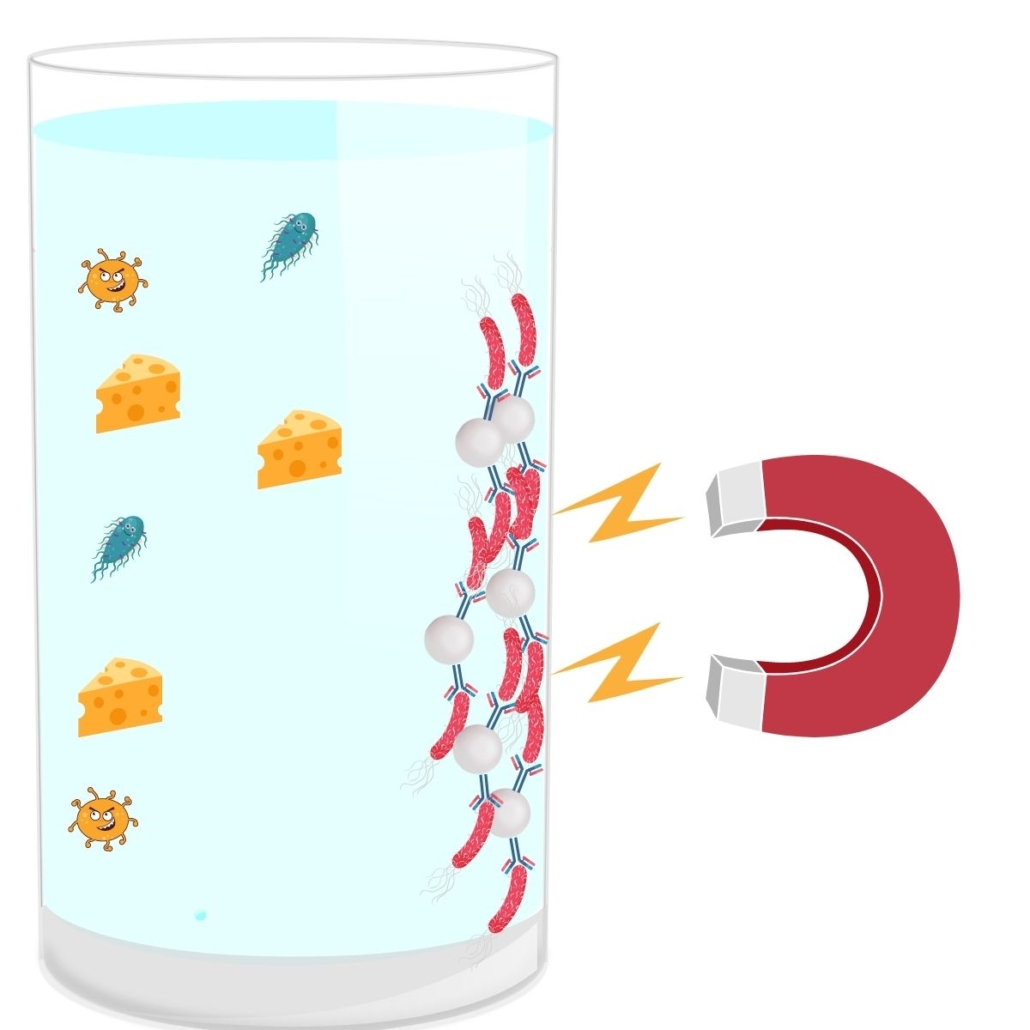
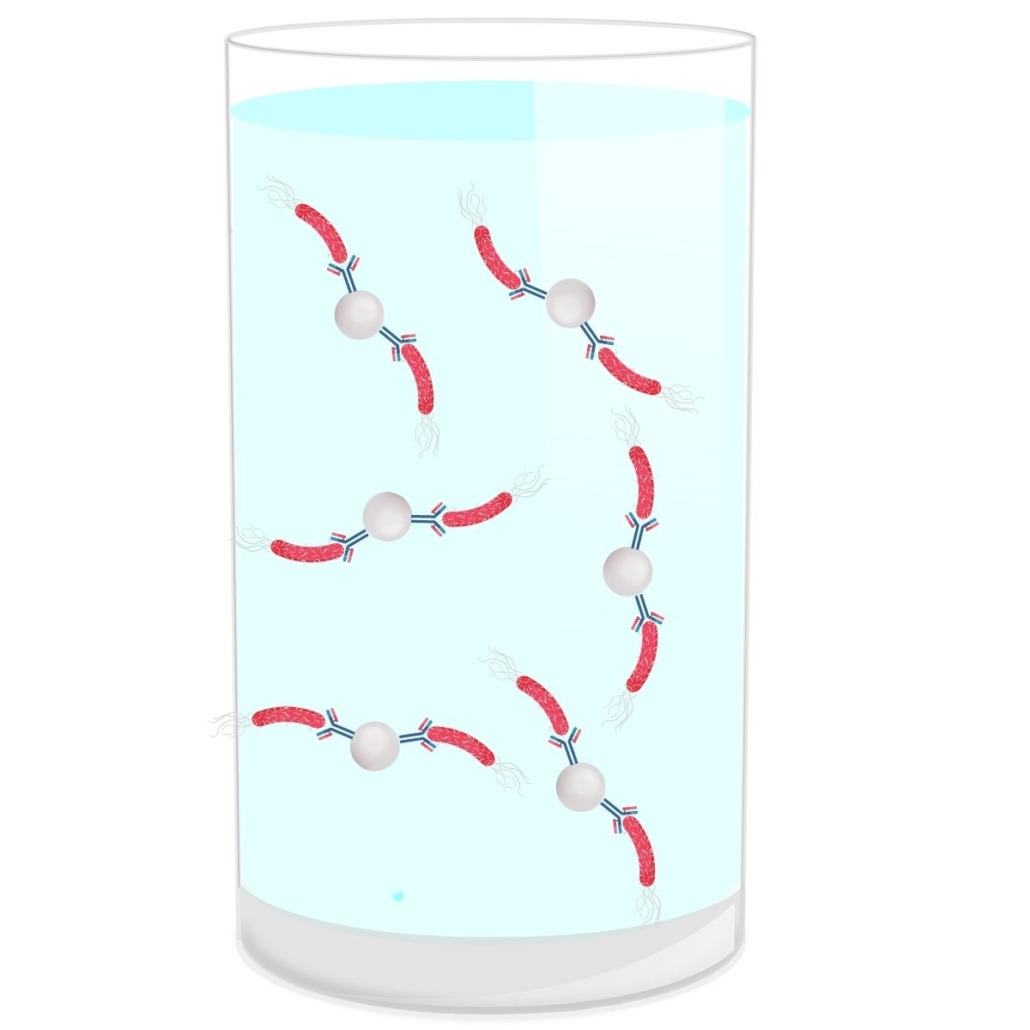
Advantages of Immunocapture:
- First Sorting: This step eliminates all serotypes that are not part of the Top 5 → reduction in the number of presumptive positives.
- Rinsing: After capture, the bacteria are rinsed. This removes the matrix and thus potential interferences and inhibitions during the PCR step (reduction in the number of false negatives). This step also eliminates DNA released by dead bacteria (reduction in false positives).
Disadvantages of Immunocapture:
- An Additional Step: This step is not complicated and can be automated, but it remains an extra step.
- Caution for Export: In some countries, this method may not be compatible with local regulations. For example, Germany only searches for stx+ genes.
Advice: Ask the supplier what their method proposes to reduce the number of presumptive positives.
Droplet Digital PCR Technology
This technology creates tiny droplets with the sample. Droplets are so tiny that there is only 1 bacteria per droplet. Then a PCR is run in each droplet.
If positive, it means that stx and eae are from the same bacteria. No further confirmation would be needed.
Advice: Ask the supplier what their method proposes to reduce the number of presumptive positives.
5 – Simplifying Confirmation
Regardless of the alternative method you choose, there will always be a percentage of presumptive positive results that will require confirmation.
There are two possible strategies here:
- Outsource the confirmation to an accredited laboratory.
- Perform the confirmation in your laboratory.
Be aware, if you opt for the second option, you will need a P2 or higher, or P3 laboratory to handle the samples.
Confirmation protocols can be long, laborious, and expensive. So, this step should be considered in your choice of method. The challenge of confirmation is to find the serotypes detected during the PCR (screening) and verify if they possess both stx AND eae genes.
Points to consider for confirmation:
- Which sample is used for confirmation?
- Do we start again from the incubation bag (broth + matrix) or from the pre-PCR immunocapture (which might save a step)?
- Do we start again from the incubation bag (broth + matrix) or from the pre-PCR immunocapture (which might save a step)?
- Post PCR IMS
- Is it manual or automated?
- Is it manual or automated?
- Agars for confirmation
- What are the chromogenic agars to use? Some may be more efficient than others.
- What are the chromogenic agars to use? Some may be more efficient than others.
- Number of colonies to confirm
- How many colonies per plate should be tested at most? ISO indicates 50, validated methods range between 3 and 10.
Advice: Ask suppliers to provide a clear decision tree for the method as well as the discordance protocol.
Remember… the fewer presumptive positives you have, the fewer confirmations you’ll need to perform.
6 – Ease of use
A few years ago, following an RT-PCR (real-time PCR) protocol felt like playing chemist (which we are (mostly) not)!
Fortunately for us, kit suppliers have made huge advancements, and the amount of pipetting (and thus the risk of error) has greatly decreased. However, there are differences between suppliers’ protocols, which can be a real distinguishing point between technologies.
DNA Extraction
If extraction is necessary, how long does it take? How much pipetting is required? What volumes are we talking about? The smaller the volume in microliters, the more skill is needed.
For some methods, extraction is performed directly in the thermocycler or at the time of the pre-PCR immunocapture step.
Adding PCR Reagents
The simplest scenario is when the reagents are already in the PCR tube (lyophilized). If not, what are the steps and what volumes are involved?
Advice: How much pipetting is required in total? With how many different pipettes?
7 – Time to Decision (TTD)
Today, the Time to Result is more or less the same across all validated methods. The durations of incubation and PCR are almost identical.
What will differ is the TTD (Time To Decision).
TTD is the time it takes to be able to decide regarding the release or not of the product. For STEC, given the presumptives and confirmations, this TTD is important… but it can be hard to determine since it depends on the flora of your matrices. If you have historical data, this can help you simulate the TTD for each method.
8 – Temperature and Incubation Time
Depending on the technology and the nature of your matrix, the incubation time can vary. For raw meat, it will be around 8-24h and for raw milk 18-24h (see the validation files for each method).
Regarding the incubation temperature, for STEC, everyone seems to agree on 41.5°C (less interfering flora than at 37°C).
Currently, ISO/TS 13136 indicates an incubation temperature of 37°C, but everyone we have spoken to agrees that in the next revision of the standard, the temperature will change to 41.5°C.
9 – Enrichment Broth
Some suppliers have validated their method with simple EPT (Buffered Peptone Water or BPW), others with modified EPT.
Questions to ask are:
- Is the modified broth proprietary? Who can supply it?
- Is it available in a format compatible with my lab (bags, powders, bottles, etc.)?
- Is it compatible with the detection of another strain, like Salmonella, for example? This allows the same broth to be used.
9 – PCR Cycle Sequence
Not all methods are equal in this respect. Regarding validated alternative methods, the strategies differ.
Some methods perform the 3 detections (eae, stx, top 5) with 1 PCR cycle, while others have a sequential approach in 2 or 3 steps (like the ISO method).
The advantage of the sequential approach is the cost. Only the necessary PCRs are initiated.
The disadvantage is that for positive samples, other PCR cycles must be launched. This delays the result and involves more handling.
Advice: Ask the supplier to provide a clear decision tree with the price and duration of each PCR.
If you have a history of the presence of eae and stx genes in your matrices, this can help you make simulations.
10 – Analytical Capacity and Throughput
Depending on the capacity of the thermocycler, you can run more or fewer analyses. Therefore, ensure that the number of analyses you need to perform is compatible with what the technology can offer.
The potential use of the thermocycler for confirmations should also be considered. You might even opt for purchasing two thermocyclers, much to your supplier’s delight 😉
11 – Result Analysis and Software
For the majority of methods, there’s no need to analyze curves and CT values to know if an RT-PCR is positive or negative. Algorithms handle this for us.
However, the software’s usability is still a factor to consider, which is often overlooked.
Advice: Ask the supplier to demonstrate the software. Also, inquire about the technology’s connectivity with a LIMS (Laboratory Information Management System).
Which PCR supplier to choose?
The Price
Price is often a critical factor. And in the context of STEC analysis, it’s essential not to overlook the cost of confirmation (labor and immobilization of goods).
Advice: Use your analysis history (% of stx, eae, Top 5 or 7) to best simulate your costs.
The Customer Service
A thermocycler can break down, in which case what does the supplier offer:
- Who intervenes (the brand’s customer service technician, a subcontractor, or distributor?) and how quickly?
- What do maintenance contracts include, and at what price?
The Application Support
Application support might be the most crucial aspect (note, the author of this article is a former Application Engineer).
With STEC analysis, you will sooner or later have questions about the results. The most common case is the analysis of discordant results.
Example: What to do when a serotype detected during the screening (PCR) step is not found during confirmation?
Who will be there to help you? In what language? Will support be remote, or can someone visit your site?
The Training
Firstly, there’s the training of your teams on the new method. This step is critical for what follows:
- Who will conduct this training (a salesperson, an application engineer, etc.)?
- How long will it last?
- How many people can participate?
The Validation
The ISO 16140 standard sets a framework for the validation of alternative methods. You will need to go through this when adopting your new STEC detection method:
- What support does the supplier offer?
- Do they provide a protocol?
- Will they be present for the manipulations or the statistical interpretation of the results?
The Trust
The last argument, which applies to all technologies, is… the trust you have in your supplier!
This might seem subjective, but it can be based on tangible criteria, such as:
- Is it easy to reach them by phone? Do you get directly through to them or to a voice server?
- Have you worked with them before? If not, you might want to inquire with another lab (on LinkedIn or via SuperMicrobiologists).
- Are they responsive, do they quickly provide you with a solution in case of a problem?
Ask Other STEC PCR Users
Choosing a method for STEC detection is not a trivial decision for a company. Therefore, nothing should be left to chance. Ideally, before making a decision, take the time to exchange and meet with several users of the technology.
If possible, avoid contacts provided by the supplier. You can find other users at a conference, on LinkedIn, by reactivating an old classmate, or by asking on the SuperMicrobiologists forum!
Once in contact, take the opportunity to ask for:
- The technology’s performance (are the announced performances the same as those achieved).
- The technology’s reliability.
The “drawbacks” and limits of the technology. Suppliers rarely communicate on this, yet all technologies have limits. Knowing these limits allows you not to exceed them!
Conclusion
Detecting STEC is not a piece of cake, but now you have all the cards in hand to make the best choice.
Our last piece of advice would be to conduct tests before deciding. Test your different matrices from different times of the year with the PCR method of your choice. To do this, either:
- You have the resources internally and conduct these tests in your laboratory.
- You outsource this study to an expert service laboratory (you might even be able to negotiate a little support from the PCR kit supplier!).
You now have all the information you need to choose the STEC detection method best suited to your lab – it’s up to you!

















Trackbacks & Pingbacks
[…] The challenge for alternative methods will be to limit the number of presumptive positives while avoiding false negatives. We precisely address this topic in another dedicated article “How to Choose Your STEC Detection Method?“. […]
[…] explaining what STEC is, why they are complicated to detect, and how to choose the most suitable method for your lab, here is the final step: A selection of 6 reliable PCR methods to detect […]
Leave a Reply
Want to join the discussion?Feel free to contribute!